Outsourcing has become a fundamental strategy for life science companies seeking to accelerate development and optimise operations. Whether it’s for preclinical research, clinical trials, regulatory affairs, manufacturing, or market access, outsourcing the right activities to the right partners can drive significant efficiencies.
However, when outsourcing is poorly managed, the consequences can be severe, ranging from cost overruns to delays, regulatory setbacks, and even the failure to bring a product to market.
For emerging life science companies, outsourcing decisions can be especially daunting. The landscape is complex, and choosing the right partners requires thorough oversight and strategic due diligence.
Let’s begin with mistake number 1.
Mistake #1: Lack of Due Diligence
The first and most significant mistake is failing to perform adequate due diligence when selecting outsourcing partners. Whether you’re engaging a CRO, CDMO, consultant, or any other specialist provider, ensuring that they are capable, compliant, and well-suited to your project is essential.
Outsourcing decisions are among the most critical choices an emerging life science company will make. Failure to properly vet providers can lead to:
- Increased costs: Inefficiencies or unqualified service providers
- Delays: Push your project off schedule and jeopardise timelines
- Poor quality: Outcomes that impact the overall integrity of your work
- Regulatory setbacks: Compliance issues or delays in approval
- Product not reaching market: Resulting in devastating consequences on your business
What to Look for in Your Outsourcing Partners
Regulatory frameworks like ICH Q7, Q8, Q9, and E6 impose strict guidelines on companies, and regulatory authorities will hold you accountable for any lapses in compliance. Failure to adhere to these standards can cause a project to come to a halt until the necessary corrective actions are taken.
Some common mistakes made due to inadequate due diligence include:
- Mistaking a software company for a full-service CRO, which leads to missing critical capabilities
- Choosing a CRO without verifying staff expertise, which can result in incorrect handling of clinical data or trial management
- Running a Phase 1 clinical study under an academic IND that fails to meet Good Clinical Practice (GCP) standards, necessitating costly restarts
- Selecting an EDC provider that subcontracts its services without disclosing it, leading to GDPR and GCP compliance gaps
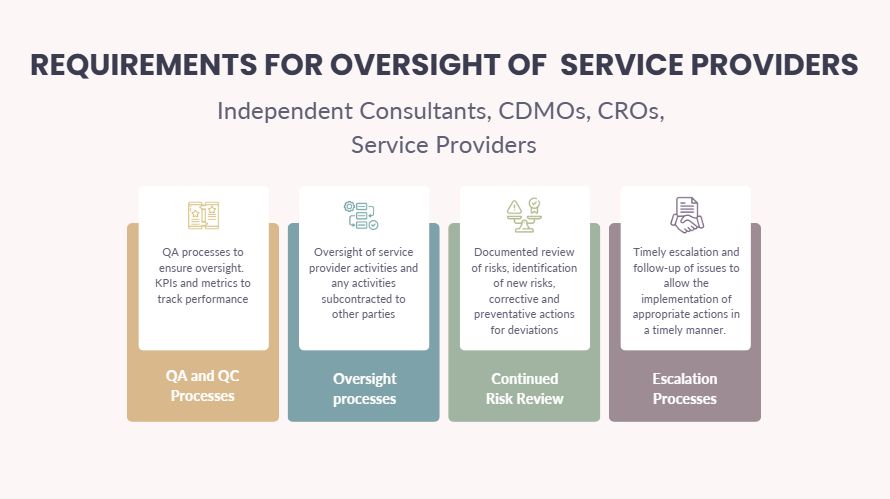
- Overlooking whether your service provider has a robust Quality Management System (QMS) that aligns with regional regulatory requirements
The Path Forward: Expert Guidance and Due Diligence
While the importance of thorough due diligence cannot be overstated, it can be difficult for emerging companies to manage this on their own, especially when resources are limited.
This is where platforms like INDICRO can provide significant value. By connecting companies with independent consultants across all R&D functions, INDICRO offers the expertise needed to:
- Help with vetting potential providers
- Ensure regulatory compliance
- Provide oversight and guidance throughout the outsourcing relationship to prevent costly mistakes
Through structured due diligence, you can make informed decisions, mitigate risks, and set your projects on the right path.
What’s Next?
In the coming weeks, we will continue to explore additional common mistakes that life science companies make when outsourcing and guide how to avoid them.
Stay tuned for Mistake #2, where we will explore the critical importance of having Standard Operating Procedures (SOPs) and a Quality Management System (QMS) in place and how we can assist you in implementing these essential frameworks.



